

溶液对实现椭偏仪在位监测电化学沉积薄膜主要会带来两方面的影响,第1种是溶液的扰动,比如在开放的溶液体系,溶液表面的扰动可能会对光产生多种散射机制,从而给测试带来困难。椭偏仪在位监测薄膜沉积过程中涉及多个界面,有空气/观察窗口界面、观察窗口/溶液界面、溶液/沉积薄膜的固液界面和薄膜/基底界面。每一个界面都会增加测试与分析的难度,如何把复杂的体系简化成为可模拟的光学模型是十分具有挑战性的。
展示全部 
椭偏仪在位表征电化学沉积的系统搭建(八)- 溶液的影响和固液界面的影响
4椭偏仪在位监测电化学沉积的挑战
椭偏仪在位监测电化学沉积的挑战主要分为:溶液的影响和固液界面的影响,以及装置的设计。
4.1溶液
溶液对实现椭偏仪在位监测电化学沉积薄膜主要会带来两方面的影响,第1种是溶液的扰动,比如在开放的溶液体系,溶液表面的扰动可能会对光产生多种散射机制,从而给测试带来困难。另外是溶液中浓度变化所带来的影响。当光波场频率很大且溶液的浓度不太大时,光学常数折射率及消光系数有如下关系式:

由朗伯定律与光强度的定义得吸收系数β与消光系数k的关系为:

又由比尔定律知,当溶液浓度足够小以至于分子间相互作用能被忽略时,溶液吸收系数β与溶液的浓度C成正比,即β=αC,α是与浓度无关由吸收物质分子的特性决定的常数。因此可以得到溶液浓度与其折射率之间的关系式为:
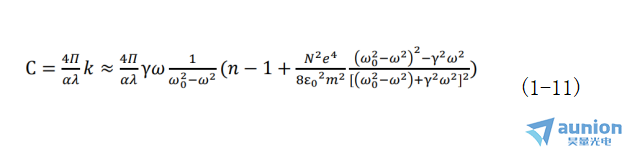
由以上推导可知光学常数n、k值和溶液浓度之间的关系如式(1-11)所示,而椭偏仪测量得到的参数ψ和Δ是光学常数n、k的函数,这意味着溶液直接影响着测试结果,不同浓度溶液带来的影响不同。所以后续研究过程中溶液以及溶液浓度对测试结果的影响都是具有挑战性的。
4.2固液界面
椭偏仪在位监测薄膜沉积过程中涉及多个界面,有空气/观察窗口界面、观察窗口/溶液界面、溶液/沉积薄膜的固液界面和薄膜/基底界面。每一个界面都会增加测试与分析的难度,如何把复杂的体系简化成为可模拟的光学模型是十分具有挑战性的。
如图1-15所示,金属电极和电解液接触面存在电压差,电子的分布会随其改变。通常情况下对于金属-电解质界面处的电荷分布如图1-15所示的简单方案外,主要取决于:1.固体的电子性质;2.水分子和水合阳离子的吸附;3.阴离子的化学吸附(表面过量);4.被应用的外部控制的电位。
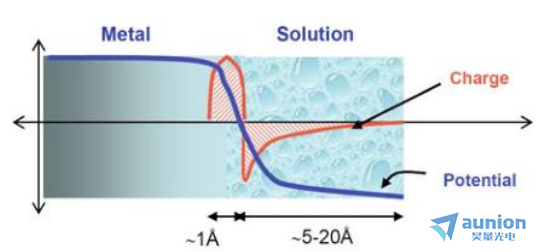
图1-15 电极与电解液界面电压及电子示意图
在沉积过程中,薄膜生长经历纳米尺度阶段,而纳米尺度的材料具有共同的电荷存储和转移能力,在简单的模型中,半导体、金属纳米粒子和分子都可以作为给体、受体或电子桥,如图1-16所示。如等离子体金属纳米粒子存在局部表面等离子体共振(LSPR)现象,它包括电子密度的耦合共振振荡和一个逐渐消失的电磁场(统称为等离子体激元),这些激元在粒子表面附近被特定波长的入射光激发。LSPR导致了特征消光(吸收加散射)波段,可能跨越紫外、可见和近红外部分的能谱。

图1-16 金属纳米粒子在半导体点和分子桥之间的电子转移的图示
因此在电化学沉积过程可能也会存在衬底与沉积物质的电荷转移现象。这些界面效应将会给椭偏测试数据的分析与提取增加难度。
了解更多椭偏仪详情,请访问上海昊量光电的官方网页:
https://www.auniontech.com/three-level-56.html
更多详情请联系昊量光电/欢迎直接联系昊量光电
关于昊量光电:
上海昊量光电设备有限公司是光电产品专业代理商,产品包括各类激光器、光电调制器、光学测量设备、光学元件等,涉及应用涵盖了材料加工、光通讯、生物医疗、科学研究、国防、量子光学、生物显微、物联传感、激光制造等;可为客户提供完整的设备安装,培训,硬件开发,软件开发,系统集成等服务。
您可以通过我们昊量光电的官方网站www.auniontech.com了解更多的产品信息,或直接来电咨询4006-888-532。
相关文献
[1] WONG H S P, FRANK D J, SOLOMON P M et al. Nanoscale cmos[J]. Proceedings of the IEEE, 1999, 87(4): 537-570.
[2] LOSURDO M, HINGERL K. ellipsometry at the nanoscale[M]. Springer Heidelberg New York Dordrecht London. 2013.
[3] DYRE J C. Universal low-temperature ac conductivity of macroscopically disordered nonmetals[J]. Physical Review B, 1993, 48(17): 12511-12526. DOI:10.1103/PhysRevB.48.12511.
[4] CHEN S, KÜHNE P, STANISHEV V et al. On the anomalous optical conductivity dISPersion of electrically conducting polymers: Ultra-wide spectral range ellipsometry combined with a Drude-Lorentz model[J]. Journal of Materials Chemistry C, 2019, 7(15): 4350-4362.
[5] 陈篮,周岩. 膜厚度测量的椭偏仪法原理分析[J]. 大学物理实验, 1999, 12(3): 10-13.
[6] ZAPIEN J A, COLLINS R W, MESSIER R. Multichannel ellipsometer for real time spectroscopy of thin film deposition from 1.5 to 6.5 eV[J]. Review of Scientific Instruments, 2000, 71(9): 3451-3460.
[7] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[8] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[9] YUAN M, YUAN L, HU Z et al. In Situ Spectroscopic Ellipsometry for Thermochromic CsPbI3 Phase Evolution Portfolio[J]. Journal of Physical Chemistry C, 2020, 124(14): 8008-8014.
[10] 焦杨景.椭偏仪在位表征电化学沉积的系统搭建.云南大学说是论文,2022.
[11] CANEPA M, MAIDECCHI G, TOCCAFONDI C et al. Spectroscopic ellipsometry of self assembLED monolayers: Interface effects. the case of phenyl selenide SAMs on gold[J]. Physical Chemistry Chemical Physics, 2013, 15(27): 11559-11565. DOI:10.1039/c3cp51304a.
[12] FUJIWARA H, KONDO M, MATSUDA A. Interface-layer formation in microcrystalline Si:H growth on ZnO substrates studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Journal of Applied Physics, 2003, 93(5): 2400-2409.
[13] FUJIWARA H, TOYOSHIMA Y, KONDO M et al. Interface-layer formation mechanism in (formula presented) thin-film growth studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Physical Review B - Condensed Matter and Materials Physics, 1999, 60(19): 13598-13604.
[14] LEE W K, KO J S. Kinetic model for the simulation of hen egg white lysozyme adsorption at solid/water interface[J]. Korean Journal of Chemical Engineering, 2003, 20(3): 549-553.
[15] STAMATAKI K, PAPADAKIS V, EVEREST M A et al. Monitoring adsorption and sedimentation using evanescent-wave cavity ringdown ellipsometry[J]. Applied Optics, 2013, 52(5): 1086-1093.
[16] VIEGAS D, FERNANDES E, QUEIRÓS R et al. Adapting Bobbert-Vlieger model to spectroscopic ellipsometry of gold nanoparticles with bio-organic shells[J]. Biomedical Optics Express, 2017, 8(8): 3538.
[17] ARWIN H. Application of ellipsometry techniques to biological materials[J]. Thin Solid Films, 2011, 519(9): 2589-2592.
[18] ZIMMER A, VEYS-RENAUX D, BROCH L et al. In situ spectroelectrochemical ellipsometry using super continuum white laser: Study of the anodization of magnesium alloy [J]. Journal of Vacuum Science & Technology B, 2019, 37(6): 062911.
[19] ZANGOOIE S, BJORKLUND R, ARWIN H. Water Interaction with Thermally Oxidized Porous Silicon Layers[J]. Journal of The Electrochemical Society, 1997, 144(11): 4027-4035.
[20] KYUNG Y B, LEE S, OH H et al. Determination of the optical functions of various liquids by rotating compensator multichannel spectroscopic ellipsometry[J]. Bulletin of the Korean Chemical Society, 2005, 26(6): 947-951.
[21] OGIEGLO W, VAN DER WERF H, TEMPELMAN K et al. Erratum to ― n-Hexane induced swelling of thin PDMS films under non-equilibrium nanofiltration permeation conditions, resolved by spectroscopic ellipsometry‖ [J. Membr. Sci. 431 (2013), 233-243][J]. Journal of Membrane Science, 2013, 437: 312..
[22] BROCH L, JOHANN L, STEIN N et al. Real time in situ ellipsometric and gravimetric monitoring for electrochemistry experiments[J]. Review of Scientific Instruments, 2007, 78(6).
[23] BISIO F, PRATO M, BARBORINI E et al. Interaction of alkanethiols with nanoporous cluster-assembled Au films[J]. Langmuir, 2011, 27(13): 8371-8376.
[24] 李广立. 氧化亚铜薄膜的制备及其光电性能研究[D]. 西南交通大学, 2016.
[25] 董金矿. 氧化亚铜薄膜的制备及其光催化性能的研究[D]. 安徽建筑大学, 2014.
[26] 张桢. 氧化亚铜薄膜的电化学制备及其光催化和光电性能的研究[D]. 上海交通大学材料科 学与工程学院, 2013.
[27] DISSERTATION M. Cellulose Derivative and Lanthanide Complex Thin Film Cellulose Derivative and Lanthanide Complex Thin Film[J]. 2017.
[28] NIE J, YU X, HU D et al. Preparation and Properties of Cu2O/TiO2 heterojunction Nanocomposite for Rhodamine B Degradation under visible light[J]. ChemistrySelect, 2020, 5(27): 8118-8128.
[29] STRASSER P, GLIECH M, KUEHL S et al. Electrochemical processes on solid shaped nanoparticles with defined facets[J]. Chemical Society Reviews, 2018, 47(3): 715-735.
[30] XU Z, CHEN Y, ZHANG Z et al. Progress of research on underpotential deposition——I. Theory of underpotential deposition[J]. Wuli Huaxue Xuebao/ Acta Physico - Chimica Sinica, 2015, 31(7): 1219-1230.
[31] PANGAROV n. Thermodynamics of electrochemical phase formation and underpotential metal deposition[J]. Electrochimica Acta, 1983, 28(6): 763-775.
[32] KAYASTH S. ELECTRODEPOSITION STUDIES OF RARE EARTHS[J]. Methods in Geochemistry and Geophysics, 1972, 6(C): 5-13.
[33] KONDO T, TAKAKUSAGI S, UOSAKI K. Stability of underpotentially deposited Ag layers on a Au(1 1 1) surface studied by surface X-ray scattering[J]. Electrochemistry Communications, 2009, 11(4): 804-807.
[34] GASPAROTTO L H S, BORISENKO N, BOCCHI N et al. In situ STM investigation of the lithium underpotential deposition on Au(111) in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide[J]. Physical Chemistry Chemical Physics, 2009, 11(47): 11140-11145.
[35] SARABIA F J, CLIMENT V, FELIU J M. Underpotential deposition of Nickel on platinum single crystal electrodes[J]. Journal of Electroanalytical Chemistry, 2018, 819(V): 391-400.
[36] BARD A J, FAULKNER L R, SWAIN E et al. Fundamentals and Applications[M]. John Wiley & Sons, Inc, 2001.
[37] SCHWEINER F, MAIN J, FELDMAIER M et al. Impact of the valence band structure of Cu2O on excitonic spectra[J]. Physical Review B, 2016, 93(19): 1-16.
[38] XIONG L, HUANG S, YANG X et al. P-Type and n-type Cu2O semiconductor thin films: Controllable preparation by simple solvothermal method and photoelectrochemical properties[J]. Electrochimica Acta, 2011, 56(6): 2735-2739.
[39] KAZIMIERCZUK T, FRÖHLICH D, SCHEEL S et al. Giant Rydberg excitons in the copper oxide Cu2O[J]. Nature, 2014, 514(7522): 343-347.
[40] RAEBIGER H, LANY S, ZUNGER A. Origins of the p-type nature and cation deficiency in Cu2 O and related materials[J]. Physical Review B - Condensed Matter and Materials Physics, 2007, 76(4): 1-5.
[41] 舒云. Cu2O薄膜的电化学制备及其光电化学性能的研究[D]. 云南大学物理与天文学院,2019.
展示全部 